MINI REVIEW
The outpost against cancer: universal cancer only markers
Chengchen Qian1, Xiaolong Zou2, Wei Li1,3, Yinshan Li4, Wenqiang Yu5
1Shanghai Epiprobe Biotechnology Co., Ltd, Shanghai 200233, China; 2 Department of General Surgery, the First Affiliated Hospital of Harbin Medical University, Harbin 150001, China; 3Shandong Epiprobe Medical Laboratory Co., Ltd, Heze 274108, China; 4 People’s Hospital of Ningxia Hui Autonomous Region, Ningxia Medical University, Yinchuan 750002, China; 5Shanghai Public Health Clinical Center & Department of General Surgery, Huashan Hospital & Cancer Metastasis Institute & Laboratory of RNA Epigenetics, Institutes of Biomedical Sciences, Shanghai Medical College, Fudan University, Shanghai 200032, China
ABSTRACT
Cancer is the leading cause of death worldwide. Early detection of cancer can lower the mortality of all types of cancer; however, effective early-detection biomarkers are lacking for most types of cancers. DNA methylation has always been a major target of interest because DNA methylation usually occurs before other detectable genetic changes. While investigating the common features of cancer using a novel guide positioning sequencing for DNA methylation, a series of universal cancer only markers (UCOMs) have emerged as strong candidates for effective and accurate early detection of cancer. While the clinical value of current cancer biomarkers is diminished by low sensitivity and/or low specificity, the unique characteristics of UCOMs ensure clinically meaningful results. Validation of the clinical potential of UCOMs in lung, cervical, endometrial, and urothelial cancers further supports the application of UCOMs in multiple cancer types and various clinical scenarios. In fact, the applications of UCOMs are currently under active investigation with further evaluation in the early detection of cancer, auxiliary diagnosis, treatment efficacy, and recurrence monitoring. The molecular mechanisms by which UCOMs detect cancers are the next important topics to be investigated. The application of UCOMs in real-world scenarios also requires implementation and refinement.
KEYWORDS
Cancer detection; cancer screening; DNA methylation; cancer epigenetics; cancer biomarkers
Why we urgently need new biomarkers?
After combatting cancer for over a century, cancer is still the most lethal biological threat to mankind. Cancer remains a global health concern with 19.3 million new cases and nearly 10 million deaths estimated in 20201. In 2020 an estimated 4.6 million new cases of cancer were diagnosed in China, accounting for 23.7% of new cancer cases globally according to GLOBOCAN1. Furthermore, approximately 3 million deaths were attributed to cancer in China in 2020, which were 30% of global cancer-related deaths1. These statistics indicated that China ranks first in the incidence and mortality rate of cancer. Moreover, the 5-year cancer survival rate is 40.5%, which is 1.5 times lower than the 5-year survival rate in the United States2,3. The comparatively lower survival and higher mortality rates in China than in countries with higher human development indexes suggest that an efficient and cost-effective cancer prevention and surveillance system is urgently needed. Early detection of cancer is one of the most critical elements in a healthcare system. Early detection of cancer can improve the prognosis and survival at an early stage in nearly all cancer types4. Successful screening strategies have led to a significant decline in the incidence and mortality rates of cervical, breast, colorectal, and prostate cancers.
To achieve an early detection of cancer, however, is not an easy task. Investigating the biology and prognosis of early cancer, identifying and validating reliable early detection bio- markers, and developing accessible and accurate early detection technologies have always been the greatest obstacles in the process4. Precise detection of cancer can distinguish benign from malignant lesions, which helps avoid unnecessary procedures and facilitates further disease management. Current early detection strategies include endoscope-based biopsies, medical imaging, cytology, immunoassays, and biomarker tests5-7. Being intrusive and costly, endoscope-based biopsies carry an inherently heavy burden as a major medical procedure relying on professional personnel. Like cytology, both screening methods depend on medical professionals and are based on personal judgment with a performance that is far from ideal8. In contrast, immunoassays are highly inaccurate, given the high false-positive rates. Medical imaging, as a screening tactic, requires expensive equipment and specialized technicians. Hence, medical imaging is extremely limited due to the low accessibility. For all these reasons, biomarkers appear to be abetter option for the early detection of cancer.
Correspondence to: Yinshan Li and Wenqiang Yu
Email: liyinshan@nxrmyy.com and wenqiangyu@fudan.edu.cn
ORCID ID: https://orcid.org/0009-0005-3340-6802 and
https://orcid.org/0000-0001-9920-1133
Received August 22, 2023; accepted October 12, 2023;
published online November 28, 2023.
Available at www.cancerbiomed.org
©2023 Cancer Biology & Medicine. Creative Commons
Attribution-NonCommercial 4.0 International License
Biomarkers are currently categorized as proteins, DNA mutation markers, epigenetic markers, chromosomal abnor- malities, RNA markers derived directly from tumors, or tumor fragments obtained indirectly from bodily fluids. Protein markers are the most widely applied biomarkers in cancer screening and diagnosis. Protein biomarkers, as screening bio- markers, are limited by the tendency to be affected by benign lesions, which leads to overdiagnosis and overtreatment, as has been reported for α-fetoprotein and prostate-specific antigen (PSA)9,10. RNA markers include genetic expression patterns and other non-coding RNA markers.A combination of genetic expression RNA markers can be detected using urine samples, the sensitivity of which was far from satisfactory (60%) for primary tumors, and the detection of which can be affected by the easy degradation nature of RNA in the normalenviron- ment11. Genetic and epigenetic markers both face the problem of prevalence in tumors and limitation to cancer types.
DNA methylation has been a strong candidate as an early detection biomarker since being first linked to can- cer by Feinberg in 198312. DNA methylation aberrations are observed in all stages of cancer, as early as the precancerous stage. Aberrant DNA hypermethylation usually takes place on CpG islands in gene promoters to counteract tumor suppressors13,14. Studies have also suggested that abnormal DNA hypermethylation engages in the upregulation of devel- opmental regulators15. The DNA methylation valley, which is commonly associated with developmental regulators and hypermethylated cancers, might switch the gene expression mode to a more stable DNA methylation-dependent mode and decrease the connection to methylated histone H3K27me3 and associated polycomb proteins16,17.
Among the large number of published DNA methylation markers, several have successfully debuted in the market; how- ever, the current commercialized DNA methylation markers and diagnostic panels have yet to fully unlock the potential of early detection of cancer for multiple reasons18. While mostly show- ing acceptable performance using database information, these biomarkers usually perform less ideally in the real world due to the fact that real-world samples are often more complex and not as representative as those selected in the databases. Next- generation-sequencing-based multi-cancer methylation early detection has been shown to have a mere 16.8% and 40.4% sen- sitivity in stage I and II cancers, respectively19. Early detection tests require greater stability and more accurate biomarkers.
Universal cancer only marker (UCOM) discovery using guide positioning sequencing (GPS)
Despite decades of cancer research, satisfactory prevention and treatment have not been realized. New methodologies are needed to enable researchers to thoroughly evaluate cancer. Over the last 23 years, 6 cancer hallmarks, such as evad- ing apoptosis, tissue invasion & metastasis, etc., have been expanded to 14 by including features like nonmutational epi- genetic reprogramming and polymorphic microbiomes20,21. As more details involving cancer are unveiled, more perspec- tives are introduced into cancer research. Cancer research has gradually come into a newera in two directions (commonality and individuality). With the development of precision oncol- ogy in recent years, the focus of cancer research is leaning towards individualized targeted therapy and the heterogeneity of cancer22. Thus, recently identified cancer biomarkers have focused mainly on specific cancer types, such as PAX6 forcer- vical cancer23 and BMP3 for colorectal cancer24. The perfor- mance of these biomarkers specific to cancer types varies, but it is still not possible for susceptible individuals to undergo screening for all cancers simultaneously due to the limitation of biological sample acquisition and the high cost. It would be ideal if we could identify a single, robust biomarker that is effective for all types of cancer at anearly stage.
To achieve such an ideal goal,a better biomarker candidate must be selected from the list of potential biomarker types. DNA methylation aberrations, among all genetic and epige- netic profiles, are known to be related to cancer and are some of the earliest, if not first, cancer-related abnormalities to occur chronologically. The investigation of DNA methylation started early, but has been hindered by the lack of research methods. Among 28 million potential methylated CpG sites in the genome, a manageable number must be detected and aligned to the genome to better understand tumorigenesis. Whole genome bisulfite sequencing (WGBS), which is consid- ered to be the gold standard of DNA methylation sequencing, can only cover 50% of Cs in cancer cells due to the nature of bisulfite treatment that breaks DNA fragments and lowers the genome complexity during the transformation of Cs-to-Ts25. Other methods, such as 450k chips, only cover 1.6% of genome methylation. Based on 450k data, a DNA methylation detec- tion panel has 35.4% sensitivity for 6 types of stage I cancers26. Limitations of cancer types, poor performance, and noise generated by detection methods in the analytic process have become the greatest obstacles for pan-cancer detection panels.
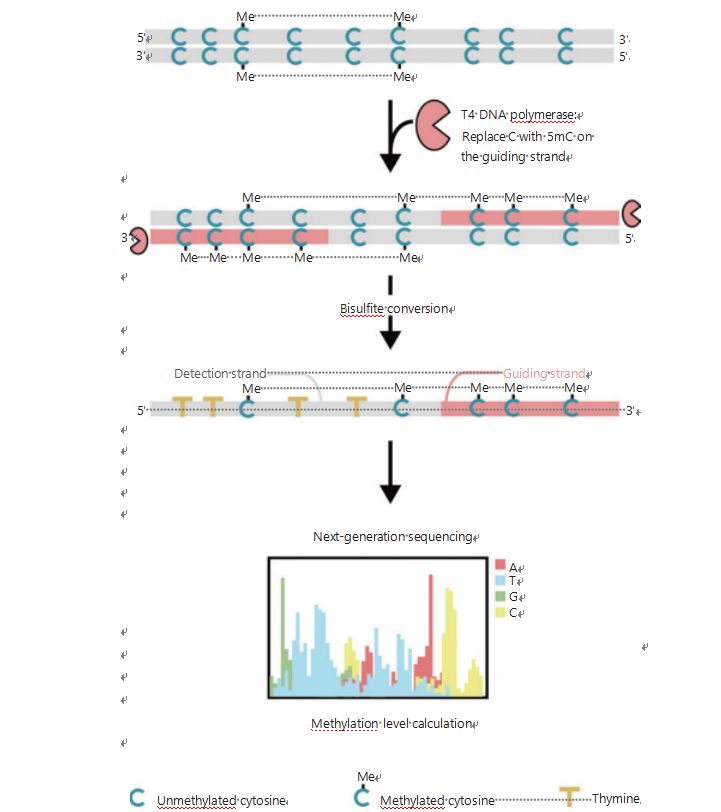
To better investigate the epigenetic patterns of cells during tumorigenesis and metastasis, we developed a unique GPS for genome-wide DNA methylation detection, which covers up to 96% of CpG sites in 0.4 billion reads25. GPS is a bilat- eral sequencing method using a 3′ end of DNA fragment of non-convertible methyl-cytosines after bisulfite treatment that guides the alignment of DNA methylation calculation of the 5′ end through pair-end sequencing (Figure 1)25. The methyl-cytosine guiding strand, acting as a template strand, aids in high-GC region alignment that recovers the most aban- doned sequencing data in traditional WGBS. The high cover- age feature of GPS provides an enormous amount of DNA methylation information, which allows us to examine cancer methylation profiles with a considerably higher resolution in previously under-investigated regions.
GPS provides us with a powerful tool to investigate the homogeneity of cancer, which can greatly simplify cancer research and potentially find a universal explanation for tum- origenesis and metastasis. While analyzing GPS data of cancer cell lines,a unique phenomenon was frequently encountered. There were a number of regions that appeared to be abnor- mally hypermethylated in multiple types of cancer samples. This unexpected finding was subsequently validated to serve as UCOMs. Greater than 7,000 samples from 17 types of cancer in The Cancer Genome Atlas (TCGA) database have been analyzed, among which we identified the first UCOM, HIST1H4F, a histone-related gene that is hypermethylated in all types of cancer27. A series of UCOMs were then found and validated in the TCGA database, the Gene Expression Omnibus (GEO) database, and real-world clinical samples. As of now, HIST1H4F, PCDHGB7, and SIX6 have been found and validated as UCOMs. The unexpected discovery of UCOMs offers a powerful answer to the need for early detection of cancer. UCOMs provide a solution for single marker detection of multiple cancers.
Characteristics of UCOMs
Upon validation, UCOMs have been shown to exhibit four major characteristics that enable UCOMs to surpass the effi- cacy of current biomarkers (Figure 2).
Unique to malignancy
UCOMs are unique to cancerous or pre-cancerous lesions and are not affected by normal physiologic changes. Some of the current cancer-related markers that have been widely applied in early detection and/or screening have led to overdiagnosis. Elevated PSA levels, a clinically accredited screening tool, are also detected in benign conditions, such as prostate hyperplasia and prostatitis10. The overdiagnosis and resulting overtreatment lead to a reduced quality of life due to bowel, urinary, and sexual complications28. Other protein-based and widely used biomarkers in the clinical setting, such as CA-125, have yielded no significant benefits while incurring overdiagnosis and overtreatment29. The high specificity of UCOMs for malignancies avoids these short- comings. The UCOM, PCDHGB7, efficiently distinguishes high-grade squamous intraepithelial lesions (HSILs) and cervical cancer from normal samples and low-grade squa- mous intraepithelial lesions (LSILs), while most other bio- markers can only differentiate cervical cancer from normal samples30. Although PCDHGB7 does not detect significant differences between normal endometrium and endometrial hyperplasia, significant differences are detected between nor- mal endometrium and atypical hyperplasia, and even greater differences are detected between normal endometrium and endometrial cancer (EC) based on PCDHGB731. UCOMs are unique to malignant lesions in databases and clinical sam- ples. From a patient’s perspective, unique UCOMs reduce the threshold for understanding complex indications of various poor-performing unstable biomarkers and the correspond- ing anxiety during the evaluation process. From the clini- cian’s perspective, unique UCOMs differentiate malignancies from benign lesions, which aid in the triage of patients and reduces unnecessary medical procedures and overtreatment. Therefore, unique UCOMs reduce medical system redun- dancy, relieve system distress, and make available more med- ical resources to those in need.
Figure 1 Schematic of GPS workflow for DNA methylation detection25. Gray line: input DNA sequence; red line: DNA treated with T4 DNA polymerase, replacing cytosine with 5′-methylcytosine at the 3′ end of the input; blue C with Me: methylated cytosine; blue C: unmethylated cytosine; yellow T: thymine25.
All or nothing
UCOMs are only present in cancer cells and are detected sta- bly in nearly all cancer cells. HIST1H4F was validated to be hypermethylated in nearly all tumor types but not in normal samples27. Similarly, PCDHGB7 and SIX6 have also been shown to be hypermethylated in all tumor samples but not in normal samples30-32. This unique characteristic significantly improves the performance of UCOMs with respect to the limit of detection and sensitivity. As few as 2% of cancer cells can be differentiated in samples, making UCOMs a much more sensi- tive biomarker than most existing biomarkers30.As a biomarker used for colorectal cancer detection, KRAS mutations only exist in approximately 36% of colorectal cancer cases, suggest- ing poor diagnostic potential33. The low prevalence of KRAS mutations in colorectal cancer limits KRAS in combination with other biomarkers. In fact,a combination of biomarkers might seem promising initially, but does not always generate a satisfactory result while demonstrating much greater noise in detection analysis and usually involves more complicated experimental procedures. In contrast, PCDHGB7 and other UCOMs exist in all cancers. UCOMs detect cancerous com- ponents in different types of cancer samples with utmost precision while eradicating complex noise-canceling analysis processes. It is not difficult to detect cancer in an abundant sample, but it is extremely challenging to detect cancer in a small sample. UCOMs are capable of detecting small amounts of cancer.
Figure 2 Characteristics of UCOMs.
Cancer detection preceding pathological changes
UCOMs can be detected in the pre-cancerous stage prior to pathological changes. As epigenetic biomarkers, UCOM abnormalities occur in an earlier stage than phenotypic abnormalities and are detectable throughout tumorigenesis, progression, and metastasis34,35. The sensitivity of UCOM over time enhances UCOM performance in detecting early-stage cancer and pre-cancerous lesions. Detection of early cancer based on biopsies and cytology can be difficult for even the most experienced pathologists. A single biopsy acquired via colposcopy has been reported as positive in 60.6% of HSIL+ samples. Additional biopsies are required for multiple lesions to increase sensitivity36. In contrast, the UCOM, PCDHGB7, has a sensitivity of 82% for HSIL+ samples, surpassing the sensitivity of biopsies and most biomarkers30. The methylation marker, FAM19A4, has a sensitivity of 69% for CIN2+, which is similar to cytology, but cannot differentiate CIN1 from normal samples37. UCOMs have been shown to be a much more sensitive early detection biomarker. Compared with expe- rience-based pathologists, UCOMs have superior detection sensitivity for early-stage cancers, which in turn contributes to improved cancer prognosis and survival30. Additionally, UCOMs offer a detection platform that is accessible to areas lacking experienced pathologists and greatly improves detection efficiency. With uniform sampling and detection proce- dures, UCOM detection yields stable and easy-to-interpret results that better suit a screening protocol requiring fewer professional personnel and medical resources.
Easy to detect
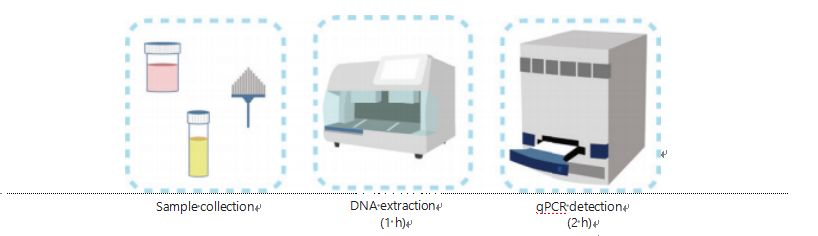
Current methods for DNA methylation detection are com- plicated and time-consuming. Most of the methods require bisulfite transformation, which causes a loss in sample quality and possibly produces unstable and inaccurate results. The poor reproducibility caused by bisulfite treatment potentially leads to confusion for physicians and patients and further interfere with the follow-up and/or treatment strategies. Therefore, we further modified the method of UCOM detection to avoid problematic bisulfite treatment of the samples, accommodate the clinical application requirements, and enhance accessibility. We developed a novel method using methylation-sensitive restriction enzymes combined with real-time fluorescent quantitative PCR (Me-qPCR) to quan- tify the methylation status of UCOMs within 3 h using easy handling procedures (Figure 3). Me-qPCR can accommodate multiple sample types, such as clinical collection of body flu- ids and self-collected urine samples. Collected clinical samples can be processed, stored, and easily proceed to detection using standardized and automated DNA extraction. The extracted DNA can then be directly applied to the Me-qPCR platform for a one-pot reaction and output quantification results. After simple result analysis using diagnostic models fitted and validated to specific cancer types, the final determination of UCOM detection results is interpreted and presented as a semi-quantitative value. The Me-qPCR platform outperforms the traditional bisulfite-pyrosequencing in UCOM detection while saving 3 h ofbisulfite conversion, according to the EZ DNA Methylation-Gold kit protocol. The innovative methylation detection platform makes UCOM detection stabler, more accurate, and more accessible30.
Figure 3 Detection process of UCOMs. Sample types include professionally sampled BALF, Pap brush, and/or self-collected urine. The DNA extraction process can be accommodated to an automatic extractor, the product of which can be directly detected by qPCR.
Application of UCOMs
Lung cancer
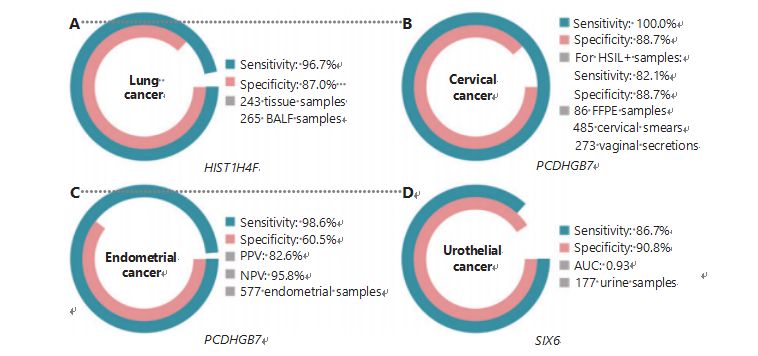
Lung cancer is the second most frequently diagnosed and most lethal cancer worldwide, accounting for 11.4% of new cases and 18.0% of new deaths1. Among all diagnoses, 85% are non-small cell lung cancer (NSCLC) and 15% are small cell lung cancer (SCLC), which has a higher level of malignancy38. Low-dose computed tomography (LDCT) scanning is the currently recommended screening method for lung cancer and has been shown to improve early detection and reduce mortality6; however, due to low specificity and poor accessibil- ity, LDCT has yet to serve as a satisfactory screening method, as do other common cancer markers, such as CEA39. The costs and potential for missed diagnoses and misdiagnoses of the LDCT screening strategy impede the progress of lung cancer screening promotion40. HIST1H4F, a UCOM, has enormous potential as an early detection biomarker in bronchoalveolar fluid (BALF) samples27. HIST1H4F is hypermethylated in lung adenocar- cinoma and lung squamous cell carcinoma, with a detection specificity of 96.7% and sensitivity of 87.0% (Figure 4A), and an exceptional performance for stage I cancers27. HIST1H4F has a specificity of 96.5% and a sensitivity of 85.4% for NSCLC, and 96.5% and 95.7%, respectively, for SCLC27. Additionally, samples of eight other types of cancer, including pancre- atic and colorectal cancers, have validated that HIST1H4F is hypermethylated in all eight types27.
Cervical cancer
Cervical cancer was the fourth most frequently diagnosed cancer and the fourth leading cause of cancer deaths in women in 2020, accounting for 3.1% of new cases and 3.4% of cancer- related deaths globally1. To eliminate cervical cancer by 2030, as proposed by the WHO, early detection of cervical cancer is a necessity. If detected at an early stage, the 5-year survival rate reaches 92% with invasive cervical cancer41. The American Cancer Society (ACS) guidelines suggest cervical cytology tests, primary HPV tests, or cotests for screening42. Cervical cytology is invasive and can only detect 63.5% of CIN2+ cases37.
PCDHGB7, in contrast, has performed much better using Pap smears and vaginal secretions, and can efficiently differentiate HSIL from LSIL in an ultra-early stage. PCDHGB7 alone has a sensitivity of 100.0% and a specificity of 88.7% for cervical cancer (Figure 4B),and an 82.1% sensitivity and 88.7% specificity for HSIL+ samples30. PCDHGB7 also has a 90.9% sensitivity and 90.4% specificity in vaginal secretion samples for cervical cancer, which are much easier to collect30. When combined with the high-risk (hr)HPV test or Thinprep Cytology Test (TCT),PCDHGB7 has an increased sensitivity of 95.7% and specificity of 96.2%, significantly surpassing that of the hrHPV test (20.3%),TCT (51.2%), and the two combined (57.8%) for cervical cancer30. PCDHGB7 has also been shown to be hypermethylated in 17 types of cancer from the TCGA database, indicating its suitability in the UCOM family30.
Figure 4 UCOMs have been validated in four types of cancer in large-scale clinical studies. A. Performance of HIST1H4F, a UCOM, in lung cancer detection of 508 samples. B. Performance of PCDHGB7, a UCOM, in cervical cancer detection of 844 samples. C. Performance of PCDHGB7, a UCOM, in endometrial cancer detection of 577 endometrial Pap and Tao brush samples. D. Performance of SIX6, a UCOM, in urothelial cancer detection of 177 samples.
EC
EC is one of the most common female reproductive system can- cers worldwide, with an estimated 4.2 million new cases and 1% of cancer-related deaths annually1. With a successful diagnosis at anearly stage, EC is curable and has a 5-year survival rate of 95% for stage I cancer. Patients who are symptomatic, such as abnormal uterine bleeding, receive periodic clinical evaluation and undergo invasive and painful biopsy procedures, despite only 5%–10% eventually developing EC43. Transvaginal ultra- sound, as the common detection method, is highly unreliable due to its inability to differentiate benign from malignant endo- metrial changes and the high false-positive rate44.
A parallel comparison of serum CA-125, a widely imple- mented EC biomarker, and PCDHGB7 was conducted. Serum CA-125 had a sensitivity of 24.8%, which suggests that CA-125 is an inadequate marker for EC despite a specificity of 92.3%31. PCDHGB7 detection using Pap brush samples yielded a sensi- tivity of 80.65% and a specificity of 82.81% for ECatall stages, while a Tao brush had a sensitivity of 61.29% and a specific- ity of 95.31%31. The PCDHGB7 diagnostic model, based on Me-qPCR, yielded a sensitivity of 98.61%, a specificity of 60.5%, and an overall accuracy of 85.5%, using Pap and Tao brush samples (Figure 4C)31.
Urothelial cancer
Urothelial cancer, consisting of bladder, renal pelvis, and ure- ter cancers, was the seventh most frequently diagnosed can- cer in 2020 worldwide, causing 5.2% of new cases and 3.9% of deaths1. Urothelial cancers, greater than 50% of which are bladder cancer, were the fourth most frequently diagnosed cancer in the United States in 2022, accounting for 11.6% of newly diagnosed cases3. Approximately 75% of bladder cancers are classified as non-muscle invasive bladder cancer restricted to the mucosa or submucosa45. A cystoscopy biopsy is the gold standard for diagnosing urothelial cancer implemented by fluorescence in situ hybridization (FISH) and cytology tests. FISH and cytology have poor diagnostic performance, and cystoscopy is intrusive and has the underlying risk of missing microlesions, misinterpreting lesions, and potentially causing a spread or relapse of cancer46. The previously validate UCOM, PCDHGB7, was also shown to be hypermethylated in urothelial cancer, with an area under the curve of 0.86, suggesting a potential diagnos- tic capability30. To further validate more UCOMs and better accommodate more sample types, SIX6, a novel UCOM, was examined and showed excellent diagnostic potential in the early detection of urothelial cancer using urine samples on the Me-qPCR platform. SIX6 detection using urine samples demonstrated a competitive sensitivity of 86.7% and a specificity of 90.8% (Figure 4D), while being non-invasive and easy to acquire32. The potential of SIX6 in metastasis monitoring and treatment efficacy evaluation is currently under investigation.
The future and challenges
UCOMs have a strong performance in the diagnostic poten- tial of multiple cancers, but there is much work left to do. We have been expanding the list of UCOMs and have been actively validating UCOMs in more types of cancer, including those that are traditionally difficult to detect. Validation results from TCGA databases have further corroborated the applica- tion of UCOMs in more types of cancer and more situations. In a preliminary investigation, UCOMs have been shown to have robust diagnostic potential for cholangiocarcinomas and pancreatic adenocarcinomas, which are nearly impossible to diagnose in anearly stage with current screening methods32,47. The ability to detect rare cancers with UCOMs can be utilized with circulating tumor DNA (ctDNA) by an improved liquid biopsy platform48. A study involving a plasma DNA-based pan-cancer detection panel yielded a sensitivity of 57.9%49. Despite the high specificity, the overall performance reveals that there is still room for improvement.
The unique characteristics of UCOMs have also supported the investigation of UCOM potential in treatment efficacy evaluation and recurrence monitoring. According to the Response Evaluation Criteria in Solid Tumours (RECIST), medical imaging is the recommended methodology for recur- rence monitoring and treatment efficacy evaluation, while tumor markers are used alone for assessment50. In reality, however, imaging approaches are greatly affected by the fre- quency and timing, and therefore expose patients to higher risk and costs51,52. SIX6 has been validated to serve as a predic- tor for breast cancer metastasis32. Liquid biopsy-based ctDNA monitoring enables real-time surveillance over minimal residual disease months ahead of radiologic detection, ideally delaying and preventing relapse-related cancer progression53. Preliminary results suggest that UCOMs reflect the level of cancerous hypermethylation in real time immediately after surgery and treatment32. The high sensitivity exhibited by UCOMs and the applicability in multiple non-intrusive sam- ple types allows UCOMs to serve as a precise recurrence mon- itoring biomarker while maintaining high patient compliance.
At the sametime, public accessibility to the test is another major issue that requires additional effort. While UCOM detection collaborations have been adopted in more hospitals in the hope of benefiting more patients, pro bono detections and screenings have been actively performed in rural China. UCOMs require improved accessibility to qualify as a feasible screening tool, especially for underdeveloped areas.
While the UCOM application results in early detection are promising, many unknowns about UCOM exist. With active exploration, additional research is warranted about why UCOMs are universally present in cancers. The under- lying epigenetic regulation mechanisms underlying UCOMs are worthy of further investigation, which could justify a new direction for cancer therapeutics. Returning to the interplay between tumor homogeneity and heterogeneity, we are inter- ested in why UCOMs can be an exception to the majority of cancer biomarkers that are tightly linked to specific cancer types. The role of UCOM-identified DNA methylation aber- rations in tumorigenesis, tumor progression, and metastasis has not been determined in the process of losing and regaining cell identity and necessitates a thorough inspection. Another major interest lies in the scope of the incorporation of the homogeneity trait of UCOMs with tissue-unique markers in the hope of approaching precise detection of cancer traces and identification of tumor tissue origins in a reverse manner. UCOMs can bean ideal tool to prevent cancer, detect cancer, and potentially defend and eliminate cancer.
Grant support
This work was supported by the National Key R&D Program of China (Grant No. 2022BEG01003), the National Natural Science Foundation of China (Grant Nos. 32270645 and 32000505), a Grant from Heilongjiang Provincial Health Commission (Grant No. 2020-111), and a Grant from Heze Science and Technology Institute (Grant No. 2021KJPT07).
Conflict of interest statement
Wei Li is the R&D Director for Shanghai Epiprobe Biotechnology Co., Ltd. Wenqiang Yu serves on the Scientific Advisory Board of Epiprobe. W. Yu and Epiprobe have approved pending patents related to this work. All other authors declare no competing interests.
Author contributions
Conceived and designed the project: Chengchen Qian and Wenqiang Yu.
Wrote the paper: Chengchen Qian.
Made the illustrations: Chengchen Qian.
Reviewed and edited the manuscript: Xiaolong Zou, Wei Li, Yinshan Li and Wenqiang Yu.
References
1. Sung H, Ferlay J, Siegel RL, LaversanneM, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of
incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021; 71: 209-49.
2. Xia C, Dong X, Li H, Cao M, Sun D, He S, et al. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin MedJ (Engl). 2022; 135: 584-90.
3. Siegel RL, Miller KD, WagleNS, JemalA. Cancer statistics, 2023. CA Cancer J Clin. 2023; 73: 17-48.
4. Crosby D, BhatiaS, Brindle KM, Coussens LM, Dive C, Emberton M, et al. Early detection of cancer. Science. 2022; 375: eaay9040.
5. Ladabaum U, Dominitz JA, KahiC, Schoen RE. Strategies for
colorectal cancer screening. Gastroenterology. 2020; 158: 418-32.
6. Tanoue LT, Tanner NT, Gould MK, Silvestri GA. Lung cancer screening. Am J Respir Crit Care Med. 2015; 191: 19-33.
7. Bouvard V, WentzensenN, Mackie A, Berkhof J, BrothertonJ, Giorgi-Rossi P, et al. The IARC perspective on cervical cancer screening. N EnglJ Med. 2021; 385: 1908-18.
8. Xue P, Ng MTA, QiaoY. The challenges of colposcopy for cervical cancer screening in LMICs and solutions by artificial intelligence. BMC Med. 2020; 18: 169.
9. Johnson P, Zhou Q, Dao DY, Lo YMD. Circulating biomarkers in the diagnosis and management of hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2022; 19: 670-81.
10. Van PoppelH, Albreht T, Basu P, HogenhoutR, CollenS, Roobol M. Serum PSA-based early detection of prostate cancer in Europe and globally: past, present and future. Nat Rev Urol. 2022; 19:
562-72.
11. HolyoakeA, O’Sullivan P, Pollock R, Best T, Watanabe J, KajitaY,
et al. Development of a multiplex RNA urine test for the detection and stratification of transitional cell carcinoma of the bladder. Clin Cancer Res. 2008; 14: 742-9.
12. Feinberg AP, Vogelstein B. Hypomethylation distinguishes genes of some human cancers from their normal counterparts. Nature. 1983; 301: 89-92.
13. Ng JM, Yu J. Promoter hypermethylation of tumour suppressor genes as potential biomarkers in colorectal cancer. IntJ Mol Sci. 2015; 16: 2472-96.
14. Esteller M. Cancer epigenomics: DNA methylomes and histone- modification maps. Nat Rev Genet. 2007; 8: 286-98.
15. Nishiyama A, Nakanishi M. Navigating the DNA methylation landscape of cancer. Trends Genet. 2021; 37: 1012-27.
16. Xie W, Schultz MD, ListerR, Hou Z, Rajagopal N, Ray P, et al. Epigenomic analysis of multilineage differentiation of human embryonic stem cells. Cell. 2013; 153: 1134-48.
17. Li Y, Zheng H, Wang Q, Zhou C, WeiL, Liu X, et al. Genome-wide analyses reveal a role of Polycomb in promoting hypomethylation of DNA methylation valleys. Genome Biol. 2018; 19: 18.
18. Koch A, JoostenSC, Feng Z, de Ruijter TC, DrahtMX, MelotteV,
et al. Analysis of DNA methylation in cancer: location revisited. Nat Rev Clin Oncol. 2018; 15: 459-66.
19. KleinEA, Richards D, Cohn A, TummalaM, Lapham R, Cosgrove D, et al. Clinical validation of a targeted methylation-based multi- cancer early detection test using an independent validation set. Ann Oncol. 2021; 32: 1167-77.
20. Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000; 100: 57-70.
21. Hanahan D. Hallmarks of cancer: new dimensions. Cancer Discov. 2022; 12: 31-46.
22. Schwartzberg L, Kim ES, Liu D, Schrag D. Precision oncology: who, how, what, when, and when not? Am Soc Clin Oncol Educ Book. 2017: 160-9.
23. Liu H, Meng X,Wang J. Realtime quantitative methylation
detection of PAX1 gene in cervical cancer screening. IntJ Gynecol Cancer. 2020; 30: 1488-92.
24. Imperiale TF, RansohoffDF, Itzkowitz SH, Levin TR, Lavin P, Lidgard GP, et al. Multitargetstool DNA testing for colorectal- cancer screening. N EnglJ Med. 2014; 370: 1287-97.
25. Li J, Li Y, Li W, Luo H, Xi Y, Dong S, et al. Guide positioning
sequencing identifies aberrant DNA methylation patterns that alter cell identity and tumor-immune surveillance networks. Genome
Res. 2019; 29: 270-80.
26. Gao Q, LinYP, Li BS, Wang GQ, Dong LQ, Shen BY, et al. Unintrusive multi-cancer detection by circulating cell-free DNA methylation sequencing (THUNDER): development and independent validation studies. Ann Oncol. 2023; 34: 486-95.
27. Dong S, Li W, Wang L, Hu J, Song Y, Zhang B, et al. Histone-related genes are hypermethylated in lung cancer and hypermethylated
HIST1H4F could serve asa pan-cancer biomarker. Cancer Res. 2019; 79: 6101-12.
28. HeijnsdijkEA, Wever EM,AuvinenA, Hugosson J, Ciatto S, Nelen V, et al. Quality-of-life effects of prostate-specific antigen screening. N EnglJ Med. 2012; 367: 595-605.
29. LuzakA, Schnell-Inderst P, Bühn S, Mayer-Zitarosa A, Siebert U. Clinical effectiveness of cancer screening biomarker tests offered as self-pay health service: a systematic review. Eur J Public Health. 2016; 26: 498-505.
30. Dong S, Lu Q,Xu P, Chen L, Duan X, Mao Z, et al.
Hypermethylated PCDHGB7 as a universal cancer only marker and its application in early cervical cancer screening. Clin Transl Med. 2021; 11: e457.
31. Yuan J, Mao Z, Lu Q,Xu P, Wang C, Xu X, et al. Hypermethylated PCDHGB7 as a biomarker for early detection of endometrial cancer in endometrial brush samples and cervical scrapings. Front Mol Biosci. 2022; 8: 774215.
32. Dong S, Yang Z,Xu P, Zheng W, Zhang B, Fu F, et al. Mutually
exclusive epigenetic modification on SIX6 with hypermethylation for precancerous stage and metastasis emergence tracing. Signal Transduct Target Ther. 2022; 7: 208.
33. Huang L, Guo Z,Wang F, Fu L. KRAS mutation: from undruggable to druggable in cancer. Signal Transduct Target Ther. 2021; 6: 386.
34. Belinsky SA, Nikula KJ, PalmisanoWA, MichelsR, SaccomannoG, GabrielsonE, et al. Aberrant methylation of p16(INK4a) is anearly event in lung cancer and a potential biomarker for early diagnosis. Proc Natl Acad Sci U SA. 1998; 95: 11891-6.
35. Robertson KD. DNA methylation and human disease. Nat Rev Genet. 2005; 6: 597-610.
36. WentzensenN, Walker JL, Gold MA, Smith KM, ZunaRE,
Mathews C, et al. Multiple biopsies and detection of cervical cancer precursors at colposcopy. J Clin Oncol. 2015; 33: 83-9.
37. De Strooper LM, Meijer CJ, Berkhof J, Hesselink AT, Snijders
PJ, Steenbergen RD, et al. Methylation analysis of the FAM19A4
gene in cervical scrapes is highly efficient in detecting cervical
carcinomas and advanced CIN2/3 lesions. Cancer Prev Res (Phila). 2014; 7: 1251-7.
38. Thai AA, Solomon BJ, Sequist LV, Gainor JF, Heist RS. Lung cancer. Lancet. 2021; 398: 535-54.
39. Grunnet M, Sorensen JB. Carcinoembryonic antigen (CEA) as tumor marker in lung cancer. Lung Cancer. 2012; 76: 138-43.
40. Wood DE, KazerooniEA, Baum SL, EapenGA, EttingerDS, Hou L, et al. Lung Cancer Screening, Version 3.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2018; 16: 412-41.
41. American Cancer Society. Cancer facts & figures. Atlanta, GA, USA: American Cancer Society; 2023 [updated 2023 March 1; cited 2023 August 22].
42. FonthamETH, Wolf AMD, Church TR, EtzioniR, Flowers CR,
Herzig A, et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer J Clin. 2020; 70: 321-46.
43. Clarke MA, Long BJ, Del Mar MorilloA, Arbyn M, Bakkum-Gamez JN, Wentzensen N. Association of endometrial cancer risk with postmenopausal bleeding in women: a systematic review and meta- analysis. JAMA Intern Med. 2018; 178: 1210-22.
44. Jacobs I, Gentry-MaharajA, Burnell M, ManchandaR, Singh N,
Sharma A, et al. Sensitivity of transvaginal ultrasound screening
for endometrial cancer in postmenopausal women: a case-control study within the UKCTOCS cohort. Lancet Oncol. 2011; 12: 38-48.
45. BabjukM, Burger M, CompératEM, Gontero P, MostafidAH,
PalouJ, et al. European Association of Urology Guidelines on Non- muscle-invasive Bladder Cancer (TaT1 and Carcinoma In Situ) -
2019 Update. Eur Urol. 2019; 76: 639-57.
46. Aragon-Ching JB. Challenges and advances in the diagnosis, biology, and treatment of urothelial upper tract and bladder carcinomas. Urol Oncol. 2017; 35: 462-4.
47. Rizvi S, KhanSA, Hallemeier CL, Kelley RK, Gores GJ.
Cholangiocarcinoma – evolving concepts and therapeutic strategies. Nat Rev Clin Oncol. 2018; 15: 95-111.
48. Ye Q, Ling S, Zheng S, Xu X. Liquid biopsy in hepatocellular
carcinoma: circulating tumor cells and circulating tumor DNA. Mol Cancer. 2019; 18: 114.
49. Zhang Y, Yao Y, Xu Y, Li L, Gong Y, Zhang K, et al. Pan-cancer
circulating tumor DNA detection in over 10,000 Chinese patients. Nat Commun. 2021; 12: 11.
50. Eisenhauer EA, Therasse P, BogaertsJ, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009; 45: 228-47.
51. LitièreS, Collette S, de Vries EG, Seymour L, BogaertsJ. RECIST - learning from the past to build the future. Nat Rev Clin Oncol.
2017; 14: 187-92.
52. Seymour L, BogaertsJ, Perrone A, FordR, Schwartz LH, Mandrekar S, et al. iRECIST: guidelines for response criteria for use in trials
testing immunotherapeutics. Lancet Oncol. 2017; 18: e143-52.
53. PantelK, Alix-Panabières C. Liquid biopsy and minimal residual disease – latest advances and implications for cure. Nat Rev Clin Oncol. 2019; 16: 409-24.
Cite this article as: Qian C, Zou X, Li W, Li Y, Yu W. The outpost against cancer: universal cancer only markers. Cancer Biol Med. 2023; 20: 806-815.
doi: 10.20892/j.issn.2095-3941.2023.0313
Post time: May-07-2024