TAGMe DNA Methylation Detection Kits (qPCR) for Urothelial Cancer
PRODUCT FEATURES
Precision

Validated over 3500 clinical samples in double-blind multi-center studies, the product has a specificity of 92.7% and a sensitivity of 82.1%.
Convenient

The original Me-qPCR methylation detection technology can be completed in one step within 3 hours without bisulfite transformation.
Non-invasive

Only 30 mL of urine sample is required to detect 3 types of cancer, including renal pelvis cancer, ureteral cancer, bladder cancer at the same time.
Application scenarios
Auxiliary Diagnosis
Population suffering from painless hematuria/ suspected of having urothelial (ureteral cancer/ renal pelvis cancer)
Cancer Risk Assessment
Surgery/ chemotherapy-requiring population with urothelial carcinoma;
Recurrence Monitoring
Postoperative population with urothelial carcinoma
INTENDED USE
This kit is used for in vitro qualitative detection of hypermethylation of the Urothelial Carcinoma(UC) gene in urothelial specimens. A positive result indicates an increased risk of UC, which requires further cystoscope and/or histopathological examination. On the contrary, negative test results indicate that the risk of UC is low, but the risk cannot be completely excluded. Final diagnosis should be based on cystoscope and/or histopathological results.
DETECTION PRINCIPLE
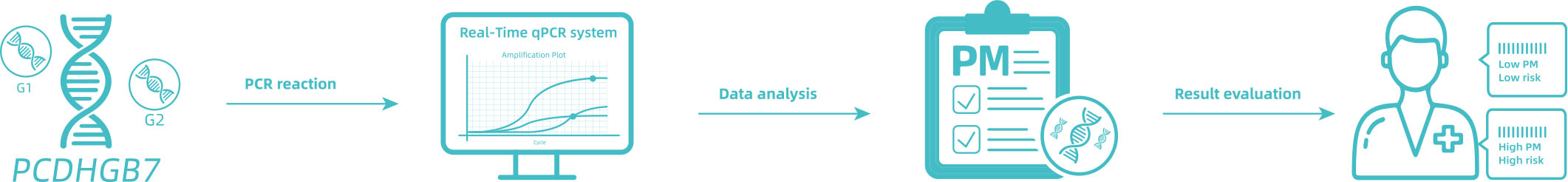
This kit contains nucleic acid extraction reagent and PCR detection reagent. Nucleic acid is extracted by magnetic-bead-based method. This kit is based on the principle of fluorescence quantitative PCR method, using methylation-specific real-time PCR reaction to analyze template DNA, and simultaneously detect the CpG sites of UC gene and the quality control marker internal reference gene fragments G1 and G2. The methylation level of UC gene, termed as Me value, is calculated according to the UC gene methylated DNA amplification Ct value and the Ct value of the reference. The UC gene hypermethylation positive or negative status is determined according to the Me value.
DNA Methylation Detection Kits (qPCR) for Urothelial Cancer

|
Clinical application |
Clinical auxiliary diagnosis of urothelial cnacer; surgery/chemotheray treatment efficacy assessment; postoperative recurrence monitoring |
|
Detection gene |
UC |
|
Sample type |
Urine exfoliated cell sample (urine sediment) |
|
Test method |
Fluorescence quantitative PCR technology |
|
Applicable models |
ABI7500 |
|
Packing specification |
48 tests/kit |
|
Storage Conditions |
Kit A should be stored at 2-30 ℃
Kit B should be stored at -20±5℃ Valid for up to 12 months. |